Abstract
Diffuse large B cell lymphoma (DLBCL) is an aggressive but potentially curable malignancy; however, cure is highly dependent on the ability to deliver intensive, anthracycline-based chemoimmunotherapy. Advanced age is an important adverse feature in prognostic models, and nearly one third of cases occur in patients older than 75 years. There is no clear accepted standard of care for older patients with DLBCL due to under-representation of this group in randomized clinical trials. R-miniCHOP, a dose attenuated version of standard R-CHOP, is often chosen based on a prospective, single-arm phase II trial which showed a 2-year progression-free survival (PFS) of 47% (Peyrade, 2011), far lower than observed outcomes in patients younger than 80 yrs (Coiffier, 2002).
Precise identification of older DLBCL patients who should be considered for curative versus palliative chemoimmunotherapy is difficult at an individual level; the challenge is to avoid both overtreatment with excessive toxicity and undertreatment with inferior outcomes. The FIL (Fondazione Italiana Linfomi; Italian Lymphoma Foundation) has developed an assessment tool accounting for age, comorbidities, and ability to perform activities of daily living (ADLs) and instrumental activities of living (IADLs). The FIL Tool classifies patients as "fit," "unfit," or "frail." When the FIL tool was prospectively applied to 1163 patients, 55% were fit, 28% were unfit, and 18% were frail. Three-year OS for the whole cohort was 65% and was strongly driven by fitness: 3-year OS was 75% for fit patients, 58% for unfit, and 43% for frail; similar outcomes were seen in a validation cohort (Merli F, 2021). However, treatment was not uniformly directed and was up to the treating physician.
Epigenetic deregulation is a feature of DLBCL in older patients, and provides a rationale for targeting methylation. Pre-clinical models show that pre-treatment with hypomethylating agents improves the anti-tumor effect of R-CHOP (Clozel T, 2013). This work has led to early clinical studies of the hypomethylating agent azacitidine with R-CHOP in newly diagnosed DLBCL, with promising early efficacy and acceptable toxicity. The availability of oral azacitidine is an appealing additive approach and is agnostic to cell-of-origin.
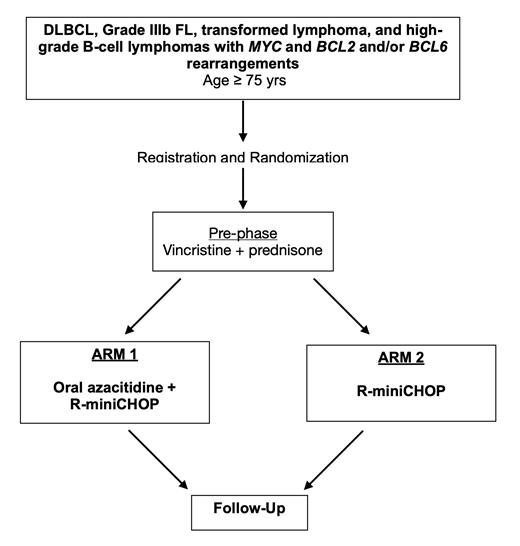
Based upon the need for improved outcomes in older patients and the promise of azacitidine in this setting, the National Clinical Trials Network (NCTN), led by SWOG, developed S1918, a randomized trial comparing R-miniCHOP to R-miniCHOP + oral azacitidine for older patients (> age 75) with newly diagnosed aggressive B-cell non-Hodgkin lymphomas. This study incorporates the FIL Tool for baseline frailty assessment and a serial comprehensive geriatric assessment (CGA) to evaluate effects of therapy on quality of life and functional status. This is the first randomized study in this population conducted in North America by the NCTN and will enroll a total of 384 eligible patients after a safety run-in phase. All patients will receive pre-phase therapy with vincristine 1mg x 1 and prednisone 60mg/m2 x 7 days (capped at 100mg/day); pre-phase therapy has been shown to improve performance status and decrease early treatment-related mortality (Owens CN, 2015).
The primary objective of the phase II component is to determine if oral azacitidine + R-miniCHOP regimen should be tested further (Phase III) against R-miniCHOP alone based on estimates of 1-year progression-free survival (PFS). The primary objective of the phase III component is to compare the OS at 2 years between oral azacitidine + R-miniCHOP and R-miniCHOP alone. Key correlative tests will include circulating tumor DNA (ctDNA) assays at pre-specified timepoints to explore if ctDNA quantity and methylation patterns correlate with response.
S1918 has the potential to impact future trial design and to change the standard of care for patients 75 years and older with aggressive lymphoma in a number of ways given its randomized design, incorporation of geriatric assessments, and utilization of ctDNA correlatives. The prospective assessment of frailty, serial comprehensive geriatric assessments, and introduction of epigenetic modulation will hopefully improve outcomes for this population with significant need.
Trial registration: ClinicalTrial.gov Identifier NCT04799275
Funding: NIH/NCI/NCTN grants U10CA180888, U10CA180819, U10CA180820, and U10CA180821
Brem: Morphosys/Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics/Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Consultancy; SeaGen: Speakers Bureau; KiTE Pharma: Membership on an entity's Board of Directors or advisory committees. Caimi: Seattle Genetics: Consultancy; Verastem: Consultancy; XaTek: Patents & Royalties: Royalties from patents (wife); Amgen Therapeutics.: Consultancy; Genentech: Research Funding; Kite Pharmaceuticals: Consultancy; ADC Theraputics: Consultancy, Research Funding; TG Therapeutics: Honoraria. Cerchietti: Bristol Myers Squibb: Research Funding; Celgene: Research Funding. Alizadeh: Foresight Diagnostics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Forty Seven: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; CAPP Medical: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Cibermed: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Roche: Consultancy, Honoraria; Janssen Oncology: Honoraria; Celgene: Consultancy, Research Funding; Gilead: Consultancy; Bristol Myers Squibb: Research Funding. Olin: Amgen: Honoraria; Cellectis: Research Funding; Pfizer: Research Funding; Daiichi Sankyo: Research Funding; Genentech: Research Funding; Abbvie: Honoraria; Actinium: Honoraria; Astellas: Honoraria, Research Funding. Friedberg: Novartis: Other: DSMC ; Bayer: Other: DSMC ; Acerta: Other: DSMC . Smith: Celgene, Genetech, AbbVie: Consultancy; Alexion, AstraZeneca Rare Disease: Other: Study investigator.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal